The Forward Reaction Is Best Described as
N2g 3H2g 2NH3g Explain in terms of collision theory why the rate of the forward reaction decreases when the concentration ofNžg is decreased. NO g Cl2 g NOCl2 g-The rate of the forward reaction increases and the rate of the reverse reaction decreases-The rate of the forward reaction decreases and the rate of the reverse reaction increases.

8 2 Chemical Equilibrium Chemistry Libretexts
Which of the following terms best describes the forward reaction in Figure 81.
. Compare the rate of the forward reaction to the rate of the reverse reaction for this system. Which best describes the rates of the forward and reverse reactions as the system approaches equilibrium. If the equilibrium of a reaction is said to lie to the right that reaction could be described as reactant- favored.
D The forward reaction is endothermic and the reverse reaction can be either endothermic or exothermic. The reaction proceeds as the substrates are converted into products View the full answer Transcribed image text. C exergonic G 0.
Given the equation representing a reaction at equilibrium. Up to 24 cash back 18. 2 The forward reaction is exothermic and the reverse Δ ΔS Δ ΔS 3.
Which statement best describes this reaction. While hypothetical reactions 1 and 2 appear to reach a point where the reaction has stopped you should imagine that reactions are still happening even after equilibrium has been reached. Usually but not always in the form of heat.
7 Which of the following terms best describes the forward reaction in the figure. The activation energy is 10 kJ and the reaction is endothermic. 4 The additional bromine ions.
Exothermic reaction in which energy is absorbed D. Endothermic reaction in which energy is absorbed 38. Forward reaction is a reaction in which products are produced from reactants and it goes from left to right in a reversible reaction.
Which statement best describes general equilibrium. Chemistry questions and answers. That is in example 2 at equilibrium J is.
B endergonic G 0. Which statement best describes the reaction. 8 HIV is the virus that causes AIDS.
The term endothermic process describes a process or reaction in which the system absorbs energy from its surroundings. Which letter represents the change in enthalpy for the reaction. The reaction is exothermic only at high temperatures.
At equilibrium the forward and reverse reactions are just happening at the same rate. Exothermic reaction in which energy is released C. It can be exhibited from the fact that the free energy of the reaction is decreased from substrate to product.
Naturally the reverse or backwards reaction is in the opposite direction. Endothermic reaction in which energy is released B. Exergonic G 0.
At equilibrium the concentration of the reactants must equal the concentration of the products. Mar 15 2022. The activation energy is 50 kJ and the reaction is exothermic.
In the mid-1990s researchers discovered an enzyme in HIV called protease. Reactant and product concentrations in the test tube are different from those in the cell. O At equilibrium the rate of the forward reaction is the same as the rate of the reverse reaction.
If the forward reaction is bond breaking then the reaction is endothermicif the forward reaction is bond formation then the reaction is exothermic. The forward reaction is exothermic and the reverse reaction can be either. At equilibrium the rate of the forward reaction equals the rate of the reverse reaction.
Which statement correctly describes the energy changes that occur in the forward reaction. D exergonic G 0. C The forward reaction is exothermic and the reverse reaction can be either exothermic or endothermic.
1 The rate of the forward reaction equals the rate of the reverse reaction. The activation energy is 50 kJ and the reaction is endothermic. Start studying the Bio Chapter 6 flashcards containing study terms like Enzymes are described as catalysts which means that they _____ Which of the following is NOT a way in which an enzyme can speed up the reaction that it catalyzes Consider a situation in which the enzyme is operating at.
O Equilibrium is reached when the reaction stops. The forward reaction is best described as an A. The reaction is exothermic in the reverse reaction.
The activation energy is 10 kJ and the reaction is exothermic. A potential energy of the reactants only. A endergonic G 0.
1 The forward reaction is exothermic and the reverse reaction is always exothermic. Up to 24 cash back Base your answer on the potential energy diagram of a chemical reaction. View the full answer.
The forward reaction is exothermic and the reverse reaction is always exothermic. The correct answer is option 1 Because equilibrium is achieved when rate of forward reaction. Further the delta G value of the given chemical reaction is negative as the given graph describes the use of an enzyme to lower the activation energy and an enzyme can only catalyse the reaction which is thermodynamically favourable.
3 by adding water h2O. Which of the following best describes the forward reaction shown in the figure. The forward reaction is exothermic and the reverse reaction is always endothermic.
2 More liquid water molecules will change to water vapor until a new equilibrium is reached. Which of the following is the best explanation for this observation. The reaction is exothermic in the forward reaction.
The forward reaction is the reaction as written towards products in the direction of the reaction arrow. Memorize flashcards and build a practice test to quiz yourself before your exam. Which statement best describes this reaction.
The reaction is endothermic in the forward reaction. A B C D heat Which statement best describes this reaction.

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

8 3 Le Chatelier S Principle Chemistry Libretexts
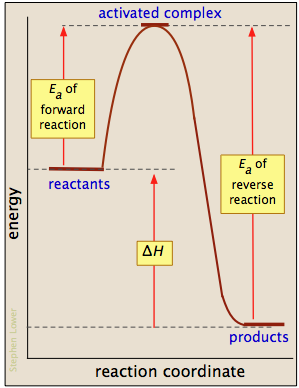
If Reaction A B Is Exothermic How Does The Activation Energy For The Forward Reaction Compare With The Activation Energy For The Reverse Reaction B A Socratic
No comments for "The Forward Reaction Is Best Described as"
Post a Comment